Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial
M.Reck1I.Bondarenko2A.Luft3P.Serwatowski4F.Barlesi5R.Chacko6M.Sebastian7H.Lu8J.-M.Cuillerot8T.J.Lynch9
ABSTRACT
Background
Ipilimumab, an anti-CTLA4 monoclonal antibody, demonstrated survival benefit in melanoma with immune-related (ir) adverse events (irAEs) managed by the protocol-defined guidelines. This phase 2 study evaluated ipilimumab + paclitaxel (Taxol)/carboplatin in extensive-disease-small-cell lung cancer (ED-SCLC).
Design
Patients (n = 130) with chemotherapy-naïve ED-SCLC were randomized 1: 1: 1 to receive paclitaxel (175 mg/m2)/carboplatin (area under the curve = 6) with either placebo (control) or ipilimumab 10 mg/kg in two alternative regimens, concurrent ipilimumab (ipilimumab + paclitaxel/carboplatin followed by placebo + paclitaxel/carboplatin) or phased ipilimumab (placebo + paclitaxel/carboplatin followed by ipilimumab + paclitaxel/carboplatin). Treatment was administered every 3 weeks for a maximum of 18 weeks (induction), followed by maintenance ipilimumab or placebo every 12 weeks. End points included progression-free survival (PFS), irPFS, best overall response rate (BORR); irBORR, overall survival (OS), and safety.
Results
Phased ipilimumab, but not concurrent ipilimumab, improved irPFS versus control [HR (hazard ratio) = 0.64; P = 0.03]. No improvement in PFS (HR = 0.93; P = 0.37) or OS (HR = 0.75; P = 0.13) occurred. Phased ipilimumab, concurrent ipilimumab and control, respectively, were associated with median irPFS of 6.4, 5.7 and 5.3 months; median PFS of 5.2, 3.9 and 5.2 months; median OS of 12.9, 9.1 and 9.9 months. Overall rates of grade 3/4 irAEs were 17, 21 and 9% for phased ipilimumab, concurrent ipilimumab and control, respectively.
Conclusion
These results suggest further investigation of ipilimumab in ED-SCLC.
Keywords
combination therapy
first-line treatment
ipilimumab
paclitaxel/carboplatin
randomized phase 2 trial
small-cell lung cancer
introduction
Combination chemotherapies are the current standard of care for extensive-disease-small-cell lung cancer (ED-SCLC) [1, 2]. Despite high initial tumor responses to these therapies, recurrence occurs rapidly with median overall survival (OS) ranging from 8 to 11 months [1, 3]. Phase 3 trials over the past 30 years evaluating the combinations of cisplatin or carboplatin with different third generation cytotoxic agents as first-line treatment have shown no incremental improvement in OS [1, 3], underscoring the need for novel treatment strategies.
Cytotoxic T-lymphocyte antigen-4 (CTLA-4), a negative regulator of T-cell activation, is an anticancer target of current interest [4., 5., 6., 7.]. Ipilimumab, a fully human anti-CTLA-4 monoclonal antibody, blocks the interaction of CTLA-4 with its ligands (CD80/CD86). This blockade augments T-cell activation and proliferation, leading to tumor infiltration by T-cells and tumor regression [8., 9., 10., 11., 12., 13., 14.]. Early clinical trials with ipilimumab have shown durable tumor responses in multiple cancer types [15., 16., 17., 18.]. Two phase 3 studies have demonstrated ipilimumab as the first agent to significantly improve OS in patients with metastatic melanoma [19, 20]. Adverse events (AEs) occurring in ≥15% of patients included pruritus, rash and diarrhea, and most AEs were managed by the drug-specific treatment guidelines [19, 20].
SCLC patients with immune-mediated Lambert-Eaton myasthenic syndrome appeared to stay longer in limited disease state and have improved survival [21]. Long-term survivors of SCLC were found to have a higher ratio of antitumor effectors T cells to regulatory T cells compared with those who had recurrent disease [22]. These observations suggest that agents such as ipilimumab that act by promoting antitumor immunity may delay tumor growth in patients with SCLC. This randomized phase 2 study was undertaken to evaluate the activity of ipilimumab in combination with paclitaxel/carboplatin versus paclitaxel/carboplatin alone in patients with ED-SCLC. The rationale for combining ipilimumab with chemotherapy was based on preclinical studies showing that chemotherapeutics induce the release of tumor-specific antigens initiating T-cell activation and sensitize tumor cells to T-cell-mediated killing [23., 24., 25., 26., 27., 28., 29.], and that certain chemotherapeutics enhance the antitumor activity of an anti-CTLA-4 monoclonal antibody [30, 31].
Paclitaxel/carboplatin was chosen as comparator in this study, because this combination is a common standard of care for non-small-cell lung cancer (NSCLC) patients, the larger of the two cohorts in this trial [32, 33], and also because the long-term efficacy of paclitaxel/carboplatin in ED-SCLC was comparable with that of cisplatin/etoposide, the commonly used chemotherapy combination in ED-SCLC [3, 34, 35].
Since the timing of chemotherapy administration relative to immunotherapy has been shown to affect outcome [29, 36, 37], ipilimumab was combined with carboplatin/paclitaxel using two alternate regimens. In the concurrent regimen, ipilimumab was administered concurrently with paclitaxel/carboplatin, allowing ipilimumab to be present at the earliest phase of chemotherapy-induced antigen release. In the phased regimen, paclitaxel/carboplatin alone was administered followed by ipilimumab/paclitaxel/carboplatin, allowing for antigen release to occur before ipilimumab exposure.
The study enrolled patients with ED-SCLC as well as those with advanced NSCLC, and was designed to analyze each cohort separately. Results for the ED-SCLC cohort are presented here. The NSCLC cohort is reported elsewhere [38].
patients and methods
study design and treatment
In this international, randomized, double-blind, multicenter phase 2 study, 334 patients were stratified by disease type (ED-SCLC, n = 130; NSCLC, n = 204) and study site. Patients in each stratum were randomized 1: 1: 1 to receive either concurrent-ipilimumab regimen (four doses of ipilimumab/paclitaxel (Taxol)/carboplatin followed by two doses of placebo/paclitaxel/carboplatin); phased-ipilimumab regimen (two doses of placebo/paclitaxel/carboplatin followed by four doses of ipilimumab/paclitaxel/carboplatin); or control regimen (up to six doses of placebo/paclitaxel/carboplatin). Ipilimumab (10 mg/kg) or placebo, paclitaxel (175 mg/m2) and carboplatin (area under the curve = 6) were administered intravenously once every 3 weeks for a maximum of 18 weeks during the induction phase, starting on day 1; the paclitaxel dose was chosen based on a phase 2 study showing that the response rate and survival with 175 mg/m2 did not differ significantly from those with 225 mg/m2, with 175 mg/m2 showing better safety [32]. Patients without progression who continued to tolerate treatment received either ipilimumab (phased- and concurrent-ipilimumab arms) or placebo (control arm) once every 12 weeks as maintenance until progression, death or intolerance.
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the ethics committee at each participating center. All patients provided written informed consent before enrollment (ClinicalTrials.gov identifier: NCT 00527735).
patients
This report focuses on the ED-SCLC cohort. Eligible patients had histologically or cytologically confirmed ED-SCLC with bidimensionally measurable lesions, were ≥18 years of age, received no prior systemic therapy for lung cancer and had an Eastern Cooperative Oncology Group performance status of 0 or 1. Key exclusion criteria included: prior treatment with CD137 agonist or CTLA-4 modulators; uncontrolled malignant pleural effusion; brain metastases; prior or current paraneoplastic syndromes related to SCLC; current malignancies or previous malignancies within 5 years; autoimmune disease; ≥grade 2 peripheral neuropathy; inadequate hematologic, hepatic or renal function; chronic use of immunosuppressive agents and/or systemic corticosteroids (except when used as premedication for paclitaxel infusion or for the management of toxic effect).
assessments
Tumor assessments using radiologic imaging were carried out at screening and every 6 weeks during the induction phase and every 12 weeks in the maintenance phase. A blinded Independent Radiologic Review Committee assessed tumor response using both modified World Health Organization (WHO) criteria (mWHO) and newly proposed immune-related (ir) response criteria (irRC) [39, 40]. The irRC represents a modification of mWHO to capture the unique tumor response patterns to ipilimumab that include regression of index lesions in the face of new lesions and initial progression (mWHO) followed by tumor stabilization or decrease in tumor burden [40]. To account for these patterns, the irRC used total tumor burden obtained by adding index lesions and measurable new lesions in determining tumor response; changes in non-index or non-measurable lesions were not considered. ir responses were defined as follows: ir-complete response (irCR), complete disappearance of all lesions; ir-partial response (irPR), ≥50% decrease of total tumor burden from baseline ir progressive disease (irPD), ≥25% increase of total tumor burden from nadir and ir-stable disease (irSD), all other settings including a slow steady decline in total tumor burden from baseline. Details of the irRC are described elsewhere [40]. Responses by both irRC and mWHO criteria were confirmed by two evaluations at least 4 weeks apart. Assessments were performed until investigator-documented progression or unacceptable toxic effect. Patients without progression who discontinued the treatment of toxic effect were followed until they received alternative therapy or withdrew consent.
Clinical laboratory parameters, AEs and irAEs were assessed at each dosing cycle and at the end of treatment. irAE was defined as an AE that was treatment-related and consistent with an immune-mediated event. AEs and irAEs were monitored for at least 70 days after the last dose of study drugs or until any ongoing event resolved or stabilized. AEs, irAEs and laboratory values were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
The guidelines for the management of irAEs included the administration of corticosteroids (orally or intravenously), a delay in a scheduled dose, or discontinuation of therapy. No dose reductions were allowed for ipilimumab. Ipilimumab dosing was skipped in the case of ≥grade 2 non-dermatologic AEs, ≥grade 3 dermatologic AEs or ≥grade 3 hematologic abnormalities, until the event improved to ≤grade 1; if the event did not improve to ≤grade 1, treatment was discontinued permanently. Dose modifications for paclitaxel and carboplatin were made according to the package inserts/product label [41, 42]. Discontinuation or interruption of one agent did not preclude continuation of the remainder.
statistical considerations
Efficacy parameters were analyzed in all randomized patients using SAS 8.0. Safety analyses included patients who received at least one dose of any study drug. Primary end point for this study was immune-related progression-free survival (irPFS, time from randomization to irPD or death) in the NSCLC cohort [38]. Efficacy end points prespecified for the ED-SCLC cohort were all secondary and included irPFS, mWHO-PFS (the time from randomization to PD per mWHO), OS (time from randomization to death), mWHO-best overall response rate (mWHO-BORR, proportion of patients with CR or PR per mWHO), irBORR (proportion of patients with irCR or irPR), mWHO-disease control rate (mWHO-DCR, proportion of patients with CR, PR or SD per mWHO) and irDCR (proportion of patients with irCR, irPR or irSD).
Assuming an exponential distribution for irPFS, it was estimated that 86 events of 120 randomized ED-SCLC patients among three arms would provide 74% power to detect a difference in irPFS [hazard ratio (HR), 0.6; median for control, 4.5 months] with one-sided α of 0.1. Likewise, 57 deaths would provide 57% power to detect a difference in OS (HR, 0.74; median for control, 10 months). irPFS, mWHO-PFS and OS were compared between each ipilimumab arm and the control arm using an unstratified log-rank test with one-sided α of 0.1. No multiplicity adjustments to α were made. No analysis by study site was conducted. irPFS, mWHO-PFS and OS were estimated using the Kaplan–Meier product limit method; 95% confidence intervals (CIs) for medians were calculated according to Brookmeyer and Crowley [43]. HRs with 95% CIs were estimated for irPFS, mWHO-PFS and OS using an unstratified Cox proportional hazards model. irBORR, BORR, irDCR and DCR were estimated, and 95% CIs were computed according to Clopper and Pearson [44]. All 95% CIs were two-sided. The patient follow-up continued beyond the initial database lock for an updated OS analysis.
results
patient disposition and characteristics
One hundred and thirty patients with previously untreated ED-SCLC were randomized between June 2008 and August 2009 in 32 centers across seven countries, and all but two patients (reasons not reported) were treated (Figure 1). The initial database lock took place on 27 August 2010 with a minimum follow-up of 11.1 months. At this lock, all patients, except for two in the concurrent-ipilimumab and one in the control arms, were off treatment. The main reason for treatment discontinuation was disease progression. As shown in supplementary Table S1, available at Annals of Oncology online, baseline demographics and disease characteristics were generally balanced across arms, except for patients aged over 65 years (control, 20%; concurrent-ipilimumab arm, 19%; phased-ipilimumab arm, 31%), and patients with elevated lactate dehydrogenase levels (control, 42%; concurrent ipilimumab, 58%; phased ipilimumab, 48%).
Figure 1. A disposition of extensive-disease-small-cell lung cancer (ED-SCLC) patients in Study CA184-041 as of 24 August 2010. Patients were randomized to either control regimen (up to six doses of placebo/paclitaxel/carboplatin), concurrent-ipilimumab regimen (four doses of ipilimumab/paclitaxel/carboplatin followed by two doses of placebo/paclitaxel/carboplatin), or phased-ipilimumab regimen (two doses of placebo/paclitaxel/carboplatin followed by four doses of ipilimumab/paclitaxel/carboplatin). Patients who discontinued treatment may have differentially discontinued one or more study therapy and may have received paclitaxel and/or carboplatin as subsequent therapy. ‘Completed induction phase’ indicates that a patient completed induction phase without entering maintenance phase. The disposition of non-small-cell lung cancer (NSCLC) patients in this study can be found (Lynch et al. [38].
treatment exposure
The median number of ipilimumab doses administered over the entire treatment period (induction plus maintenance) was four in both the concurrent-ipilimumab (range, 1–12 doses) and phased-ipilimumab (1–7 doses) arms. Each ipilimumab-containing arm had 12 patients (29%) receiving five or more doses of ipilimumab. The median number of paclitaxel doses administered was five in the concurrent-ipilimumab (range, 1–6) and six in both the phased-ipilimumab (range, 2–6) and control (range, 1–6) arms. The median number of carboplatin doses given was six in both the control (range, 1–6) and phased-ipilimumab (range, 2–6) arms, and five in the concurrent-ipilimumab arm (range, 1–6). The number of patients entering the maintenance phase was 14 (32%) in the control, 12 (29%) in the concurrent-ipilimumab and 15 (36%) in the phased-ipilimumab arms. The median number of ipilimumab doses received in the maintenance phase was 1.5 (range, 1–8) for concurrent-ipilimumab and 1 (range, 1–3) for phased-ipilimumab arms, with the median number of placebo in the control arm being 1 (range, 1–5).
efficacy
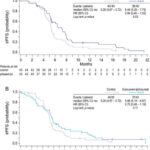
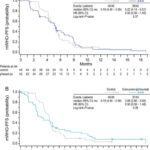
Progression was assessed using both the irRC criteria (see Patients and Methods) and modified WHO criteria. The phased-ipilimumab regimen improved irPFS compared with control (HR, 0.64; P = 0.03, Figure 2), while the concurrent-ipilimumab regimen did not (HR, 0.75; P = 0.11). Median irPFS was 5.3 months for control, 6.4 months for phased-ipilimumab and 5.7 months for concurrent-ipilimumab regimens (Figure 2). mWHO-PFS was similar across arms with the medians being 5.2 months for control, 5.2 months for phased ipilimumab and 3.9 months for concurrent ipilimumab (Figure 3).
Figure 2. Kaplan–Meier plots for progression-free survival (PFS) per immune-related (ir) response criteria (irPFS). To account for the unique tumor response patterns to ipilimumab, immune-related response criteria (irRC) was proposed. Per irRC, new lesions, whether measurable or not, were not considered progression. Measurable new lesions were rather added to the index lesions to obtain total tumor burden, and a ≥25% reduction in this total tumor burden from nadir was defined as immune-related progression. irPFS was defined as the time from the randomization to immune-related progression [as determined by an Independent Radiologic Review Committee (IRRC)] or death. As indicated by symbols, patients who neither progressed nor died were censored on the date of last tumor assessment. P-values are based on an unstratified log-rank test with a one-sided α of 0.1.
Figure 3. Kaplan–Meier plots for progression-free survival per modified WHO criteria (mWHO-PFS). Per mWHO, a reduction in index lesions by ≥25%, any new lesions (measurable or not) or progression of non-index lesions were considered an mWHO progression. mWHO-PFS was defined as the time from the randomization to mWHO progression (as determined by an IRRC) or death. As indicated by symbols, patients who neither progressed nor died were censored on the date of last tumor assessment. P-values are based on an unstratified log-rank test with a one-sided α of 0.1.
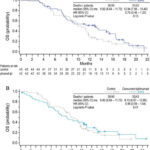
At the initial analysis (data cut-off; 27 August 2010) with 99 deaths (76%) reported, median OS for phased ipilimumab was 12.9 months, an increase of 3.0 months over the median of 9.9 months for control (HR, 0.75; P = 0.13; Figure 4). Median OS for concurrent ipilimumab was 9.1 months. In a follow-up OS analysis with 114 deaths (88%) reported (data cut-off: 5 January 2011), median OS was 10.5 months (95% CI, 8.6–11.7) for control, 12.5 months (95% CI, 7.9–14.9) for phased ipilimumab and 9.1 months (95% CI, 6.7–13.0) for concurrent ipilimumab. The HR values relative to control were 0.76 (95% CI, 0.48–1.19) for phased ipilimumab and 0.89 (95% CI, 0.57–1.39) for concurrent ipilimumab.
Figure 4. Kaplan–Meier plots for overall survival (OS). OS was defined as the time from randomization until death from any cause. As indicated by symbols, patients who had not died or who were lost to the follow-up were censored on the last date on which they were known to have been alive. Data cut-off for this analysis was 27 August 2010. P-values are based on an unstratified log-rank test with a one-sided α of 0.1.
Tumor response rates appeared to favor phased ipilimumab, but not concurrent ipilimumab, over control, the differences being greater when assessed by irRC (Table 1). irBORR was 53% for control, 49% for concurrent ipilimumab (including one irCR) and 71% for phased ipilimumab; corresponding mWHO-BORRs were 49, 33 (including one CR) and 57%, respectively (Table 1). irDCRs for control, concurrent-ipilimumab and phased-ipilimumab regimens were 96, 81 and 93%, respectively. Corresponding mWHO-DCRs were 93, 70 and 81%, respectively. In the control arm, response rates by irRC were similar to those by mWHO criteria.
Table 1. Tumor response and disease control
| Response | Control (n = 45) | Concurrent ipilimumab (n = 43) | Phased ipilimumab (n = 42) |
| ir-BOR, n (%) | |||
| irCR | 0 | 1 (2) | 0 |
| irPR | 24 (53) | 20 (47) | 30 (71) |
| irSD | 19 (42) | 14 (33) | 9 (21) |
| irPD | 0 | 0 | 2 (5) |
| Unknown | 2 (4) | 8 (19) | 1 (2) |
| irBORR, % (95% CI) | 53 (38, 68) | 49 (33, 65) | 71 (55, 84) |
| irDCR, % (95% CI) | 96 (85, 100) | 81 (67, 92) | 93 (81, 99) |
| mWHO-BOR, n (%) | |||
| CR | 0 | 1 (2) | 0 |
| PR | 22 (49) | 13 (30) | 24 (57) |
| SD | 20 (44) | 16 (37) | 10 (24) |
| PD | 0 | 5 (12) | 8 (19) |
| Unknown | 3 (7) | 8 (19) | 0 |
| mWHO-BORR, % (95% CI) | 49 (34, 64) | 33 (19, 49) | 57 (41, 72) |
| mWHO-DCR, % (95% CI) | 93 (82, 99) | 70 (54, 83) | 81 (66, 91) |
ir, immune related; BOR, best overall response; CR, complete response; PR, partial response; SD, stable disease, PD, progressive disease; BORR, best overall response rate; DCR, disease control rate; mWHO, modified WHO; CI, confidence interval.
safety
As shown in Table 2, the overall incidence of treatment-related grade 3/4 AEs appeared more common in ipilimumab-containing arms (concurrent, 43%; phased, 50%) than in the control arm (30%). However, rates of AE-related discontinuation were similar across arms (control, 9%; concurrent, 7%; phased, 5%; Figure 1).
Table 2. Adverse events and laboratory abnormalities
| Events, % | Control (n = 44) | Concurrent ipilimumab (n = 42) | Phased ipilimumab (n = 42) | ||||||
| Grade 1/2 | Grade 3 | Grade 4 | Grade 1/2 | Grade 3 | Grade 4 | Grade 1/2 | Grade 3 | Grade 4 | |
| Any adverse event | 39 | 34 | 9 | 24 | 38 | 7 | 26 | 36 | 17 |
| Any treatment-related adverse event | 61 | 23 | 7 | 41 | 36 | 7 | 45 | 36 | 14 |
| Treatment-related non-hematologic adverse events | |||||||||
| Fatigue | 20 | 5 | 0 | 24 | 7 | 0 | 17 | 12 | 0 |
| Alopecia | 59 | n/a | n/a | 57 | n/a | n/a | 67 | n/a | n/a |
| Rash | 2 | 0 | 0 | 31 | 5 | 0 | 24 | 0 | 0 |
| Pruritus | 5 | 0 | 0 | 24 | 0 | 0 | 17 | 2 | 0 |
| Arthralgia | 32 | 0 | 0 | 24 | 0 | 0 | 36 | 10 | 0 |
| Decreased appetite | 9 | 0 | 0 | 17 | 2 | 0 | 10 | 0 | 0 |
| Diarrhea | 11 | 5 | 0 | 21 | 2 | 2 | 24 | 10 | 0 |
| Nausea | 20 | 2 | 0 | 24 | 0 | 0 | 29 | 0 | 0 |
| Peripheral neuropathy | 11 | 0 | 0 | 14 | 0 | 0 | 24 | 0 | 0 |
| Peripheral sensory neuropathya | 32 | 0 | 0 | 24 | 0 | 0 | 33 | 0 | 0 |
| Hematologic abnormalitiesb | |||||||||
| Thrombocytopenia | 61 | 2 | 0 | 44 | 3 | 0 | 50 | 5 | 2 |
| Neutropenia | 40 | 2 | 0 | 41 | 5 | 3 | 40 | 5 | 5 |
| Anemia | 84 | 7 | 0 | 87 | 5 | 0 | 81 | 5 | 5 |
| Liver function enzymesb | |||||||||
| ALT | 21 | 0 | 0 | 36 | 15 | 3 | 36 | 2 | 2 |
| AST | 33 | 0 | 0 | 39 | 10 | 3 | 33 | 7 | 0 |
Adverse events listed were those (any grade) reported in at least 15% of treated patients in any arm. Patients could have more than one adverse event.
a
As reported by investigators (standardized MedDRA query term scope).
b
Based on laboratory results; n/a, not applicable; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Some of the most common non-hematologic AEs (≥15%, any grade), including fatigue, alopecia, nausea and peripheral sensory neuropathy, were generally similar across arms in terms of frequency and severity. The other common AEs including rash, pruritus and diarrhea occurred more frequently in the ipilimumab-containing arms compared with control, and these AEs were also identified as irAEs per protocol-defined criteria. As shown in Table 2, hematologic abnormalities (any grades) were generally similar across arms, although grade 3/4 abnormalities appeared more frequent in the ipilimumab-containing arms versus control (7–10% versus 2%).
The overall incidence of grade 3/4 irAEs was 9% for control, 21% for concurrent ipilimumab and 17% for phased ipilimumab. There were no grade 4 events of rash or pruritus. All events of severe diarrhea were grade 3, except for one grade 4 event for concurrent ipilimumab. One occurrence of grade 3 colitis was recorded for phased ipilimumab. There were four cases of grade 3 arthralgia for phased ipilimumab. Grade 3/4 increases in alanine aminotransferase were noted in seven patients (grade 3 six, grade 4 one) for concurrent ipilimumab, two patients (grade 3 one, grade 4 one) for phased ipilimumab and none for control. The corresponding values for aspartate aminotransferase were five patients (grade 3 four, grade 4 one), three patients (all grade 3) and none, respectively. There were two cases of grade 4 hepatitis, one (2%) in each ipilimumab-containing arm. There was no occurrence of hypophysitis, although one grade 3 event of decreased visual acuity was reported for phased ipilimumab. One death in the concurrent-ipilimumab arm was attributed to treatment-related hepatotoxicity.
discussion
This first study of ipilimumab in patients with ED-SCLC showed that the phased-ipilimumab regimen prolonged OS numerically compared with paclitaxel/carboplatin alone (median, 12.9 versus 9.9 months; HR, 0.75; P = 0.13). There was improvement in irPFS (HR, 0.64; P = 0.03) but not in mWHO-PFS (HR, 0.93; P = 0.37). The response data based on the newly proposed irRC criteria, which have yet to be validated, do not allow firm conclusions and must be considered hypothesis generating. Retrospective analysis of data from phase 2 studies in patients with metastatic melanoma suggests that the irRC may better capture the activity of ipilimumab than the WHO criteria [18, 40, 45]. Two ongoing phase 3 trials of ipilimumab in lung cancer (ClinicalTrials.gov identifiers: NCT01285609 and NCT01450761) will evaluate prospectively if irPFS may better correlate with OS than mWHO-PFS.
No improvements in efficacy end points studied including irPFS, mWHO-PFS, OS and tumor response were noted with concurrent ipilimumab. While factors contributing to the activity seen with phased ipilimumab are unknown, it is interesting to speculate that the exposure to chemotherapy before ipilimumab may have contributed to the enhancement of antitumor immune response by ipilimumab. Studies in preclinical tumor models and in patients suggest that sequencing of chemotherapy and immunotherapy may affect clinical outcome [36, 37, 46].
The common AEs seen during the study that are typically associated with paclitaxel/carboplatin, including alopecia, fatigue, nausea and peripheral neuropathy, were generally unaffected by the addition of ipilimumab. Consistent with previous experience from other studies [20, 21], the most common irAEs involved skin (rash and pruritus) and gastrointestinal tract (diarrhea), and occurred more frequently in the ipilimumab-containing arms. Treatment-related AEs, irAEs and laboratory abnormalities were mostly grade 1/2. Overall rates of treatment-related grade 3/4 AEs appeared higher for ipilimumab-containing regimens compared with the control, as were the occurrence of alanine aminotransferase and aspartate aminotransferase elevations. Most grade 3/4 irAEs were managed by the protocol-specified treatment guidelines including close patient follow-up and the early administration of systemic corticosteroids [21, 47]. Overall, in this phase 2 trial, the combination of ipilimumab plus paclitaxel/carboplatin exhibited an acceptable safety profile in ED-SCLC patients.
A major limitation of this analysis is small study population, although given the relative rarity of ED-SCLC, the population (n = 130) studied here is a large series. The fact that irRC has not yet been validated warrants caution in interpreting the irRC-based data described here. While irRC appeared to have captured the unique patterns of ipilimumab’s tumor response in this patient cohort, only 8 of the 28 patients who had PD (mWHO) without irPD were followed until irPD, precluding a full assessment of immune-related responses. An additional limitation is the use of paclitaxel (Bristol-Myers Squibb, Princeton, New Jersey)/carboplatin as comparator, because combinations of cisplatin (Bristol-Myers Squibb, Princeton, New Jersey) or carboplatin (Bristol-Myers Squibb, Princeton, New Jersey) with etoposide (Gensia Sicor Pharmaceuticals, Inc., Irvine, California) are the most commonly used standard of care for SCLC. While, at the time this study was designed, preclinical data were available only for the combination of ipilimumab and paclitaxel, subsequent preclinical studies showed synergy between etoposide and ipilimumab [30].
In conclusion, ipilimumab in combination with paclitaxel/carboplatin appeared to show clinical activity in patients with previously untreated ED-SCLC when administered as phased but not as concurrent regimen. Clinical activity with the phased regimen was also reported in the NSCLC cohort of this study [38]. Ipilimumab did not appear to exacerbate toxic effects observed with paclitaxel/carboplatin alone, and the profile of irAEs was consistent with previous experience from other ipilimumab studies. A phase 3 trial (NCT01450761) comparing phased ipilimumab plus etoposide/platinum therapy with etoposide/platinum therapy alone in patients with newly diagnosed ED-SCLC is underway.
acknowledgements
Contributions of patients and investigators who participated in these studies are gratefully acknowledged. Writing, editorial and administrative assistance was provided by Motasim Billah, an employee of Bristol-Myers Squibb.
funding
The study was sponsored by Bristol-Myers Squibb.
disclosures
Dr Martin Reck has received honoraria from AstraZeneca, Daiichi, Merck and Roche and was on the advisory boards of AstraZeneca, Bristol-Myers Squibb, Daiichi, Lilly, Pfizer and Roche. Dr Thomas Lynch holds stock of Infinity Pharmaceuticals, receives royalties from Partners Healthcare and has received honoraria from Astex, Boehringer Ingelheim, Infinity Pharmaceuticals and Merck. All other authors have declared no conflict of interest.
references
- Puglisi, S. Dolly, A. Faria, et al.Treatment options for small cell lung cancer – do we have more choice?
Br J Cancer, 102 (2010), pp. 629-638
CrossRefView Record in ScopusGoogle Scholar
- Argiris, J.R. MurrenStaging and clinical prognostic factors for small-cell lung cancer
Cancer J, 7 (2001), pp. 437-447
View Record in ScopusGoogle Scholar
- Oze, K. Hotta, K. Kiura, et al.Twenty-seven years of phase III trials for patients with extensive disease small-cell lung cancer: disappointing results
PLoS One, 4 (2009), p. e7835
doi:10.1371/journal.pone.0007835
T.L. Walunas, D.J. Lenschow, C.Y. Bakker, et al.CTLA-4 can function as a negative regulator of T cell activation
Immunity, 1 (1994), pp. 405-413
doi:10.1016/1074-7613(94)90071-X
ArticleDownload PDFView Record in ScopusGoogle Scholar
M.F. Krummel, J.P. AllisonCD28 and CTLA-4 have opposing effects on the response of T cells to stimulation
J Exp Med, 182 (1995), pp. 459-465
View Record in ScopusGoogle Scholar
D.R. Leach, M.F. Krummel, J.P. AllisonEnhancement of antitumor immunity by CTLA-4 blockade
Science, 271 (1996), pp. 1734-1736
doi:10.1126/science.271.5256.1734
View Record in ScopusGoogle Scholar
D.B. Page, J. Yuan, J.D. WolchokTargeting cytotoxic T-lymphocyte antigen 4 in immunotherapies for melanoma and other cancers
Immunotherapy, 2 (2010), pp. 367-379
CrossRefView Record in ScopusGoogle Scholar
- Hoos, R. Ibrahim, A. Korman, et al.Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy
Semin Oncol, 37 (2010), pp. 533-546
doi:10.1053/j.seminoncol.2010.09.015
ArticleDownload PDFView Record in ScopusGoogle Scholar
- Tarhini, E. Lo, D.R. MinorReleasing the brake on the immune system: ipilimumab in melanoma and other tumors
Cancer Biother Radiopharm, 25 (2010), pp. 601-613
CrossRefView Record in ScopusGoogle Scholar
- Attia, G.Q. Phan, A.V. Maker, et al.Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4
J Clin Oncol, 23 (2005), pp. 6043-6053
View Record in ScopusGoogle Scholar
A.V. Maker, G.Q. Phan, P. Attia, et al.Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study
Ann Surg Oncol, 12 (2005), pp. 1005-1016
View Record in ScopusGoogle Scholar
J.C. Yang, M. Hughes, U. Kammula, et al.Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis
J Immunother, 30 (2007), pp. 825-830
doi:10.1097/CJI.0b013e318156e47e
View Record in ScopusGoogle Scholar
- Klein, L.M. Ebert, T. Nicholaou, et al.Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4
Clin Cancer Res, 15 (2009), pp. 2507-2513
doi:10.1158/1078-0432.CCR-08-2424
CrossRefView Record in ScopusGoogle Scholar
F.S. Hodi, G. DranoffThe biologic importance of tumor-infiltrating lymphocytes
J Cutan Pathol, 37 (Suppl 1) (2010), pp. 48-53
doi:10.1111/j.1600-0560.2010.01506.x
CrossRefView Record in ScopusGoogle Scholar
E.J. Small, N.S. Tchekmedyian, B.I. Rini, et al.A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer
Clin Cancer Res, 13 (2007), pp. 1810-1815
doi:10.1158/1078-0432.CCR-06-2318
CrossRefView Record in ScopusGoogle Scholar
S.M. Ansell, S.A. Hurvitz, P.A. Koenig, et al.Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma
Clin Cancer Res, 15 (2009), pp. 6446-6453
doi:10.1158/1078-0432.CCR-09-1339
CrossRefView Record in ScopusGoogle Scholar
F.S. Hodi, M. Butler, D.A. Oble, et al.Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients
Proc Natl Acad Sci USA, 105 (2008), pp. 3005-3010
CrossRefView Record in ScopusGoogle Scholar
S.J. O’Day, M. Maio, V. Chiarion-Sileni, et al.Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study
Ann Oncol, 21 (2010), pp. 1712-1717
ArticleDownload PDFCrossRefView Record in ScopusGoogle Scholar
F.S. Hodi, S.J. O’Day, D.F. McDermott, et al.Improved survival with ipilimumab in patients with metastatic melanoma
N Engl J Med, 363 (2010), pp. 711-723
View Record in ScopusGoogle Scholar
- Robert, L. Thomas, I. Bondarenko, et al.Ipilimumab plus dacarbazine for previously untreated metastatic melanoma
N Engl J Med, 364 (2011), pp. 2517-2526
View Record in ScopusGoogle Scholar
- Maddison, J. Newsom-Davis, K.R. Mills, et al.Favourable prognosis in Lambert-Eaton myasthenic syndrome and small-cell lung carcinoma
Lancet, 353 (1999), pp. 117-118
ArticleDownload PDFView Record in ScopusGoogle Scholar
- Tani, K. Tanaka, J. Idezuka, et al.Regulatory T cells in praneoplastic neurological syndromes
J Neuroimmunol, 196 (2008), pp. 166-169
doi:10.1016/j.jneuroim.2008.03.002
ArticleDownload PDFView Record in ScopusGoogle Scholar
R.E. Merritt, A. Mahtabifard, R.E. Yamada, et al.Cisplatin augments cytotoxic T-lymphocyte-mediated antitumor immunity in poorly immunogenic murine lung cancer
J Thorac Cardiovasc Surg, 126 (2003), pp. 1609-1617
doi:10.1016/S0022-5223(03)00707-4
ArticleDownload PDFView Record in ScopusGoogle Scholar
- Tesniere, L. Apetoh, F. Ghiringhelli, et al.Immunogenic cancer cell death: a key-lock paradigm
Curr Opin Immunol, 20 (2008), pp. 504-511
ArticleDownload PDFView Record in ScopusGoogle Scholar
- Apetoh, A. Tesniere, F. Ghiringhelli, et al.Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies
Cancer Res, 68 (2008), pp. 4026-4030
doi:10.1158/0008-5472.CAN-08-0427
CrossRefView Record in ScopusGoogle Scholar
- Correale, M.T. Del Vecchio, M. La Placa, et al.Chemotherapeutic drugs may be used to enhance the killing efficacy of human tumor antigen peptide-specific CTLs
J Immunother, 31 (2008), pp. 132-147
doi:10.1097/CJI.0b013e31815b69c8
View Record in ScopusGoogle Scholar
- Ramakrishnan, D. Assudani, S. Nagaraj, et al.Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice
J Clin Invest, 120 (2010), pp. 1111-1124
CrossRefView Record in ScopusGoogle Scholar
L.A. EmensChemoimmunotherapy
Cancer J, 16 (2010), pp. 295-303
doi:10.1097/PPO.0b013e3181eb5066
View Record in ScopusGoogle Scholar
- Zitvogel, L. Apetoh, F. Ghiringhelli, et al.Immunological aspects of cancer chemotherapy
Nat Rev Immunol, 8 (2008), pp. 59-73
CrossRefView Record in ScopusGoogle Scholar
- Masters, L. Barreto, E. Girit, et al.Antitumor activity of cytotoxic T-lymphocyte antigen-4 blockade alone or combined with paclitaxel, etoposide or gemcitabine in murine models
J Immunother, 32 (2009), p. 994
(Abstract)
- Lee, M.N. Jure-Kunkel, M.E. SalvatiSynergistic activity of ixabepilone plus other anticancer agents: preclinical and clinical evidence
Ther Adv Med Oncol, 3 (2011), pp. 11-25
CrossRefView Record in ScopusGoogle Scholar
- Kosmidis, N. Mylonakis, D. Skarlos, et al.Paclitaxel (175 mg/m2) plus carboplatin (6 AUC) versus paclitaxel (225 mg/m2) plus carboplatin (6 AUC) in advanced non-small-cell lung cancer (NSCLC): a multicenter randomized trial. Hellenic Cooperative Oncology Group (HeCOG)
Ann Oncol, 11 (2000), pp. 799-805
ArticleDownload PDFView Record in ScopusGoogle Scholar
J.H. Schiller, D. Harrington, C.P. Belani, et al.Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer
N Engl J Med, 346 (2002), pp. 92-98
CrossRefView Record in ScopusGoogle Scholar
- Gridelli, L. Manzione, F. Perrone, et al.Carboplatin plus paclitaxel in extensive small cell lung cancer: a multicentre phase 2 study
Br J Cancer, 84 (2001), pp. 38-41
View Record in ScopusGoogle Scholar
- Thomas, O. Castelnau, D. Paillotin, et al.Phase II trial of paclitaxel and carboplatin in metastatic small-cell lung cancer: a Groupe Francais de Pneumo-Cancerologie study
J Clin Oncol, 19 (2001), pp. 1320-1325
View Record in ScopusGoogle Scholar
A.K. Nowak, R.A. Lake, A.L. Marzo, et al.Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells
J Immunol, 170 (2003), pp. 4905-4913
CrossRefView Record in ScopusGoogle Scholar
S.J. Antonia, N. Mirza, I. Fricke, et al.Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer
Clin Cancer Res, 12 (2006), pp. 878-887
doi:10.1158/1078-0432.CCR-05-2013
CrossRefView Record in ScopusGoogle Scholar
- Lynch, I. Bondarenko, A. Luft, et al.Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multi-center phase 2 study
J Clin Oncol, 30 (2012), pp. 2046-2054
View Record in ScopusGoogle Scholar
A.B. Miller, B. Hoogstraten, M. Staquet, et al.Reporting results of cancer treatment
Cancer, 47 (1981), pp. 207-214
doi:10.1002/1097-0142(19810101)47
:1<207::AID-CNCR2820470134>3.0.CO;2-6
CrossRefView Record in ScopusGoogle Scholar
J.D. Wolchok, A. Hoos, S. O’Day, et al.Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria
Clin Cancer Res, 15 (2009), pp. 7412-7420
doi:10.1158/1078-0432.CCR-09-1624
CrossRefView Record in ScopusGoogle Scholar
Taxol (paclitaxel)US Full Prescribing Information
Bristol-Myers Squibb, Princeton, NJ (2010)
Paraplatin (carboplatin)US Full Prescribing Information
Bristol-Myers Squibb, Princeton, NJ (2008)
- Brookmeyer, J. CrowleyA confidence interval for the median survival time
Biometrics, 38 (1982), pp. 29-41
- Clopper, E. PearsonThe use of confidence or fiducial limits illustrated in the case of the binomial
Biometrika, 26 (1934), pp. 404-413
J.D. Wolchok, B. Neyns, G. Linette, et al.Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study
Lancet Oncol, 11 (2010), pp. 155-164
doi:10.1016/S1470-2045(09)70334-1
- Braly, C.F. Nicodemus, C. Chu, et al.The immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer
J Immunother, 32 (2009), pp. 54-65
doi:10.1097/CJI.0b013e31818b3dad
View Record in ScopusGoogle Scholar
A.M. Di Giacomo, M. Biagioli, M. MaioThe emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications
Semin Oncol, 37 (2010), pp. 499-507
doi:10.1053/j.seminoncol.2010.09.007